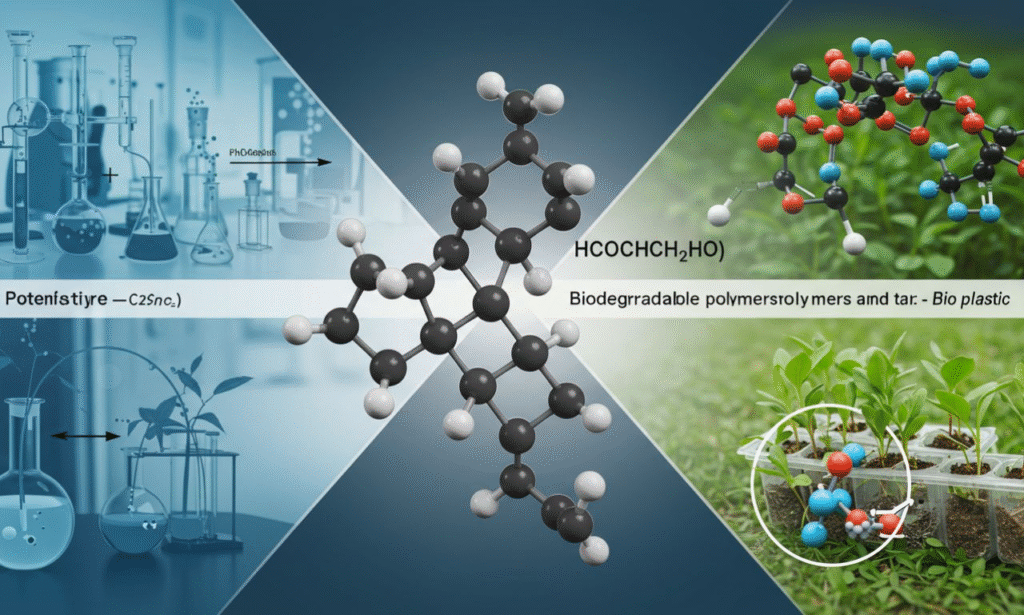
HCOOH CH2 H2O is an intriguing mix within the field of Chemistry. It is composed of the acid formic (HCOOH) and the Methylene group (CH2) and the water (H2O). The three molecules, which are simple and powerful, have a major role to play in organic chemical reactions and industrial reactions and green energy development. By understanding how the HCOOH CH 2H2O is able to behave and interact, we can comprehend its role in the production of fuels, plastics and eco-friendly chemical products.
What is HCOOH CH2 H2O?

Chemical mix HCOOHCH2H2O is a symbol of the link between formic acid, an Methylene compound and water, which is one of the major players in Organic chemical reactions. In combination, these three substances aid in the creation of esters, aldehydes and alcohols, and various organic substances..
The trio is located in the hydration, oxidation and estrification procedures and is crucial in the process of research at the universities as in industrial manufacturing processes.
Related articles: home
Breaking Down HCOOH CH2 H2O Components
1. HCOOH (Formic Acid) – The Powerful Organic Acid
Formic acid with the formula HCOOH, is considered to be the most fundamental carboxylic acid that is recognized in the field of chemistry. It is naturally present in ants and insects, and is widely used in industries.
- Utilization: Acts as a reducer and also a catalyser for acids.
- Industrial Uses: They are utilized in the manufacturing of rubber and textile finishing along with preservatives and tanning leather.
In the HCOOH H2O systems, the reactivity formic acid is responsible for reactions that lead to esters Methanol, formaldehyde and esters..
2. CH2 (Methylene Group) – The Carbon Connector
The theory is it is the CH2 group, often called as methylene, can be described as an interconnecting bridge in carbon-based molecules. While it’s not freely accessible, it can be found in other compounds, such as Methanol (CH3OH) and formaldehyde (CH2O).
In this HCOOH CH2 H2O mix Methylene is an extremely important reaction center. It participates in oxidation, polymerization or the hydrogenation process. It is essential to the formation of fuel intermediaries and organic pathways for the synthesis.
3. H2O (Water) – The Universal Solvent
Chemical reactions cannot be carried out in the absence of the use of water. When it is HCOOH and H2O reactions the water functions as an solvent and catalyst for the reaction.
- It maintains reaction balance.
- Helps in this process hydration and hydrolysis..
- Manages the temperature as well as pH in chemical syntheses.
Water’s ability to breakdown formic acids and stabilize intermediates in reaction is vital in this molecular system.
Chemical Nature and Behavior of HCOOH CH2 H2O
3-way in HCOOH H2O has the capability to react and polarity, as well as stabilization.
- Its Polarity Due to hydrogen bonds strong between formic acid and water molecules.
- Reactivity allows reduction and oxidation in normal conditions.
- Stability It is maintained at monitored conditions of pH and temperatures.
This is a great option for laboratory and industrial processing experiments and also techniques to fuel uses.
How HCOOH CH2 H2O Interacts in Chemical Reactions
If HCOOH H2O as well as HCOOH react with each other and create a variety of chemical reactions that are influenced by the surrounding conditions, for example temperature, catalyst or the concentration.
- Formic acid is proton donor.
- CH2 compound are reactive intermediates.
- The water helps in balancing and in the creation of substances.
These interactions form the base for crucial reactions such as the esterification, oxidation as well as the production of formaldehyde..
Industrial Importance of HCOOH CH2 H2O
1. Leather and Textile Processing
Formic acid found in HCOOH H2O mixtures helps in the control of pH, as well as cleaning, and stabilizes fibers making it essential for fabric and leather care.
2. Rubber Coagulation
Utilized to make naturally latex into sheets of rubber the formic acid-water solution are more secure and efficient than traditional acids.
3. Chemical Manufacturing
HCOOH CH2 H2O system allows the synthesizing of esters, aldehydes, and alcohols which are utilized for the production of solvents, perfumes and also in the manufacture of plastics.
4. Energy and Fuel Applications
Systems for Formic Acid and Water are being studied to develop the development of fuel cells to generate hydrogen which is an eco-friendly alternative to energy.
Key Reactions Involving HCOOH CH2 H2O
Esterification Reaction
A very well-known reaction that is the creation of esters:
HCOOH + CH3OH =HCOOCH3 +
In this instance formic acid is able to react with Methanol to produce the methyl formate, which is an essential solvent along with the water that is a product.
This reaction shows the balance between chemical and physical processes that occur in HCOOH as well as H2O systems.
Hydrolysis and Dehydration Reactions
HCOOHCH2H2O mixtures can be subjected to hydrolysis, which splits these molecules down into pieces, and the removal of water to form different chemical compounds. The reactions described above are widely used within the pharmaceutical and polymer industries.
Formaldehyde Formation
Under heating or catalytic conditions, formic acids as well as Methylene compounds create formaldehyde (CH2O). This is an essential step for the creation of resins, plastics, and disinfectants.

In laboratories, HCOOH CH2 H2O is frequently used to:
- Solvent technique used for extraction as well as titration.
- Catalysing agent to aid in the process of the process of oxidation or esterification.
- Blendture Model to examine acid-base as well as Redox reactants.
Chemists are attracted to this method because of its reliability in predicting Reactivity and controlled behavior in moderate conditions.
Environmental and Safety Considerations
While that HCOOH CH2 or H2O mixtures can be beneficial, formic acid is extremely destructive and must be handled with caution.
Safety Tips
- Make sure you wear eye protection as well as gloves.
- Use in a space which is aerated, such as fume hoods.
- neutralize spills using mild alkalis.
Environmental Protection
The removal of formic acid-based compounds should be governed by environmental laws in order to minimize the chance of polluting. Modern methods attempt to neutralize or reuse the byproducts in order to guarantee an environmentally safe removal of the waste.
HCOOH CH2 H2O in Modern and Green Chemistry
HCOOHCH2H2O system implements ecological chemical principles that have green and sustainable uses.
- Formic acid which is bio-based can be made by using bio-based biomass.
- Reactions made with water limit the use of harmful solvents.
- CH2 intermediators assist in the production of biodegradable materials as well in the production of fuels.
This means it is a green option for modern manufacturing of chemical products.
Future Scope of HCOOH CH2 H2O Research
Future developments include:
- Hydrogen-based fuel development via formic acid water system.
- New catalysts to accelerate, faster esterification.
- A zero-waste chemical synthesis of organic compounds.
In the course of industry moves towards sustainable growth, HCOOH CH2 H2O Chemistry will be more efficient to meet sustainable production targets.
Challenges in Storing and Using HCOOH CH2 H2O
Despite its usefulness, HCOOH CH2 H2O mixtures must be stored with caution:
- Beware of exposure to extreme temperatures or the direct light of.
- Put them in airtight, sealed containers that are resistant to acid.
- Do not touch oxidizers which are strong or base.
These precautions could aid in maintaining stable the quality of medication as well as stop unwanted reactions.
Educational Importance of HCOOH CH2 H2O

In schools and universities, HCOOH CH2 H2O is often used in studies to show:
- Acid-base equilibrium
- Esterification
- Hydrobonding and Polarity
The experiments will aid students in understanding the basic Organic Chemical concepts.
Conclusion
Chemical trio of HCOOH CH2-representing Methylene water, formic acid, and Methylene – forms an impressive system to understand the behavior of atoms during reaction. In everything from industrial manufacturing and green solutions to energy sources,, their interactions are the basis of the chemistry behind the advancement of technology.
In order to gain a deeper understanding of the chemical basis that is behind HCOOHCH2H2O, we are gaining a better understanding of its We are making progress to a sustainable and efficient scientific future.
FAQs
1. What is HCOOH CH2 uses for H2O?
It’s used in many industries, such as tanning leather, as well as chemical manufacturing and processing of rubber. It’s also utilized in research studies to create renewable fuels.
2. What makes formic acid important to HCOOH H2O?
Because it is the reaction acid that is the catalyst for essential reactions including isomerification as well as the process of the process of oxidation.
3. How does water affect the HCOOH reaction?
What role does water’s involvement within HCOOH reactions? acts as a catalyst and solvent helping to maintain the chemical equilibrium.
4. Does HCOOH CH2H2O make useful compounds?
Yes, it is able to make alcohols, esters aldehydes and esters and the fuel intermediates.
5. Are the HCOOH CH2 H2O H2O process eco green?
It is eco-friendly with biodegradable, recyclable and recyclable components.